Chapter 3 The Origin of Earth and the Solar System
Karla Panchuk
If you were to get into a time machine and visit Earth shortly after it formed (around 4.5 billion years ago), you would probably regret it. Large patches of Earth’s surface would still be molten, which would make landing your time machine very dangerous indeed. If you happened to have one of the newer time-machine models with hovering capabilities and heat shields, you would still face the inconvenience of having nothing to breathe but a tenuous wisp of hydrogen and helium gas, and depending on how much volcanic activity was going on, volcanic gases such as water vapour and carbon dioxide. Some ammonia and methane might be thrown in just to make it interesting, but there would be no oxygen. Assuming you had the foresight to purchase the artificial atmosphere upgrade for your time machine, it would all be for naught if you materialized just in time to see an asteroid, or worse yet another planet, bearing down on your position. The moral of the story is that early Earth was a nasty place, and a time machine purchase is not something to take lightly. Furthermore, there was no moon, or a magnetic field to shield you from the constant bombardment of high energy particles spewing out from our Sun.
Why was early Earth so nasty?
The early Earth was hot
Earth’s heat comes from the decay of radioactive elements within Earth, as well as from processes associated with Earth’s formation. Let’s look more closely at how those formation processes heated up Earth:
- Heat came from the thermal energy already contained within the objects that accreted to form the Earth.
- Heat came from collisions. When objects hit Earth, some of the energy from their motion went into deforming Earth, and some of it was transformed into heat. Clap your hands vigorously to experience this on a much smaller (and safer!) scale.
- As Earth became larger, its gravitational force became stronger. This increased Earth’s ability to draw objects to it, but it also caused the material making Earth to be compressed, rather like Earth giving itself a giant gravitational hug. Compression causes materials to heat up.
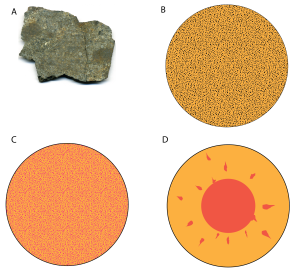
Heating had a very important consequence for Earth’s structure. As Earth grew, it collected a mixture of silicate mineral grains as well as iron and nickel. These materials were scattered throughout Earth (Figure 3.4.1B). That changed when Earth began to heat up: it got so hot that both the silicate minerals and the metals melted. The metal melt was much denser than the silicate mineral melt, so the metal melt sank to Earth’s center to become its core, and the silicate melt rose upward to become Earth’s crust and mantle (Figure 3.4.1 D). In other words, Earth unmixed itself. The separation of silicate minerals and metals into a rocky outer layer and a metallic core, respectively, is called differentiation. The movement of silicate and metal melts within Earth caused it to heat up even more. Differentiation of Earth into a planet with a metallic core was an important part in making our planet habitable, as it was a key step in generating a magnetic field.
Earth’s high temperature early in its history also means that early tectonic processes were accelerated compared to today, and Earth’s surface was more geologically active.
Earth was heavily bombarded by objects from space
Although Earth had swept up a substantial amount of the material in its orbit as it was accreting, unrest within the solar system caused by changes in the orbits of Saturn and Jupiter was still sending many large objects on cataclysmic collision courses with Earth. The energy from these collisions repeatedly melted and even vaporized minerals in the crust, and blasted gases out of Earth’s atmosphere. Very old scars from these collisions are still detectable, although we have to look carefully to see them. For example, the oldest impact site discovered is the 3 billion year old Maniitsoq “crater” in west Greenland, although there is no crater to see. What is visible are rocks that were 20 km to 25 km below Earth’s surface at the time of the impact, but which nevertheless display evidence of deformation that could only be produced by intense, sudden shock.
The evidence of the very worst collision that Earth experienced is not subtle at all. In fact, you have probably looked directly at it hundreds of times already, perhaps without realizing what it is. That collision was with a planet named Theia, which was approximately the size of Mars (Figure 3.4.2). Not long after Earth formed, Theia struck Earth. When Theia slammed into Earth, Theia’s metal core merged with Earth’s core, and debris from the outer silicate layers was cast into space, forming a ring of rubble around Earth. The material within the ring coalesced into a new body in orbit around Earth, giving us our moon. Remarkably, the debris may have coalesced in 10 years or fewer! This scenario for the formation of the moon is called the giant impact hypothesis.
Earth’s atmosphere as we know it took a long time to develop
Earth’s first experiment with having an atmosphere didn’t go well. It started out with a thin veil of hydrogen and helium gases that came with the material it accreted. However, hydrogen and helium are very light gases, and they bled off into space.
Earth’s second experiment with having an atmosphere went much better. Volcanic eruptions built up the atmosphere by releasing gases. The most common volcanic gases are water vapor and carbon dioxide (CO2), but volcanoes release a wide variety of gases. Other important contributions include sulfur dioxide (SO2), carbon monoxide (CO), hydrogen sulfide (H2S), hydrogen gas, and methane (CH4). Meteorites and comets also brought substantial amounts of water and nitrogen to Earth. It is not clear what the exact composition of the atmosphere was after Earth’s second experiment, but carbon dioxide, water vapor, and nitrogen were likely the three most abundant components.

One thing we can say for sure about Earth’s second experiment is that there was effectively no free oxygen (O2, the form of oxygen that we breathe) in the atmosphere. We know this in part because prior to 2 billion years ago, there were no sedimentary beds stained red from oxidized iron minerals. Iron minerals were present, but not in oxidized form. At that time, O2 was produced in the atmosphere when the Sun’s ultraviolet rays split water molecules apart; however, chemical reactions removed the oxygen as quickly as it was produced.
It wasn’t until well into Earth’s third experiment—life—that the atmosphere began to become oxygenated. Photosynthetic organisms used the abundant CO2 in the atmosphere to manufacture their food, and released O2 as a by-product. At first all of the oxygen was consumed by chemical reactions, but eventually the organisms released so much O2 that it overwhelmed the chemical reactions and oxygen began to accumulate in the atmosphere, although present levels of 21% oxygen didn’t occur until about 350 Ma. Today the part of our atmosphere that isn’t oxygen consists largely of nitrogen (78%).
The oxygen-rich atmosphere on our planet is life’s signature. If geologic process were the only processes controlling our atmosphere, it would consist mostly of carbon dioxide, like the atmosphere of Venus. It is an interesting notion (or a disconcerting one, depending on your point of view) that for the last 2 billion years the light reflected from our planet has been beaming a bar code out to the universe, similar to the ones in Figure 3.2.4, except ours says “oxygen.” For 2 billion years, our planet has been sending out a signal that could cause an observer from another world to say, “That’s odd… I wonder what’s going on over there.”
Media Attributions
- Physical Geology-2nd Edition, by Steven Earle is licensed under CC BY 4.0, chapter written by Karla Panchuk, and adapted by Ryan B. Anderson.
- Fig 3.4.1 A: Ordinary chondrite NWA 869 slice, James St. John, CC BY
- Fig 3.4.1: © Ryan B. Anderson, CC BY.
- Figure 3.4.2: “Planetary Smash-Up” by NASA/JPL-Caltech. Public domain.
the un-mixing of a magma, typically by the physical separation of minerals that crystallize early and settle towards the bottom
the theory that the Moon formed when a Mars-sized planet (Theia) collided with the Earth at 4.5 Ga
